What is UDI?
UDI stands for “Unique Device Identifier.” It is a unique code used to identify a medical device and its key attributes. UDI also refers to “Unique Device Identification,” which reflects the entire system that utilizes the UDI unique code.
Why is UDI Important?
The UDI system enables identification of a medical device at any time during its life cycle— from manufacturing and distribution to import, inventory release, and patient use.
The implementation of a UDI system has several beneficial uses, including:
Improves safety by enabling faster, more accurate reporting and analysis of adverse events.
Reduce medical errors through precise device identification and access to important information.
Enhance data analysis across health records, claims, and registries, strengthening postmarket surveillance and supporting premarket approval of new devices.
Streamlines recalls with a standardized identifier for better tracking by manufacturers and healthcare providers.
Strengthens global supply chain by aiding in addressing counterfeiting and preparing for medical emergencies.
Promotes international recognition of a unified device identification system.
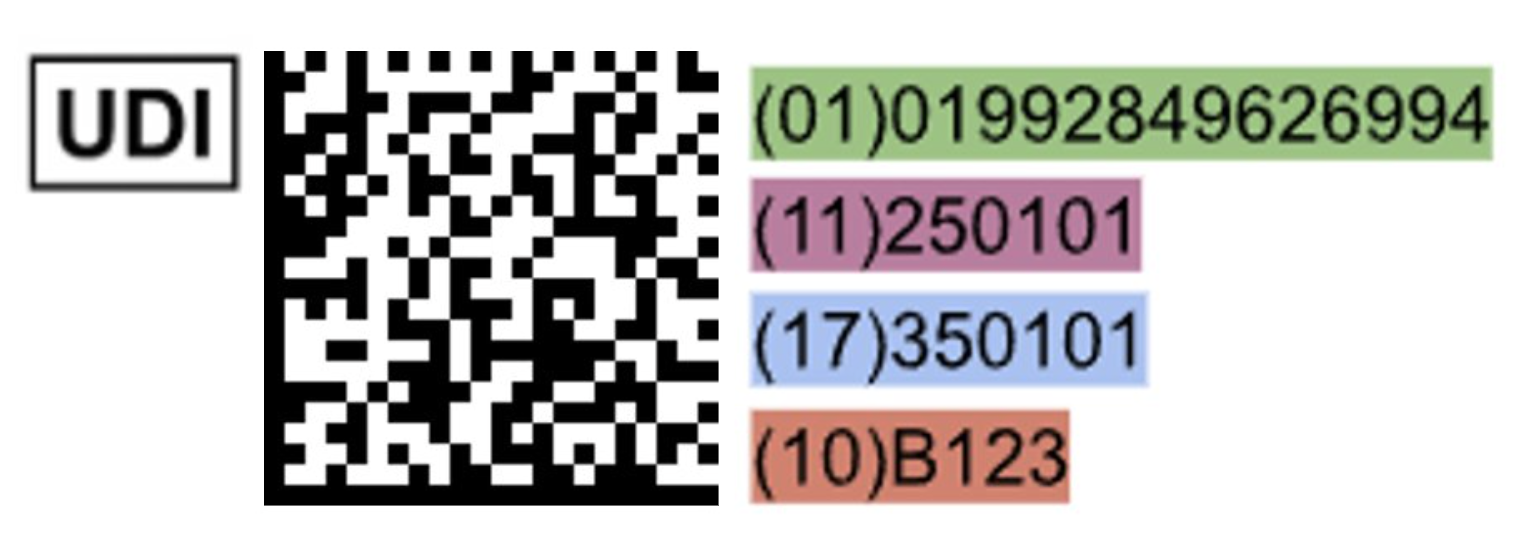
What Does UDI Look Like?
Each health authority creates its own regulations that define UDI and the UDI System, but some parameters have been globally harmonized by the International Medical Device Regulatory Forum (IMDRF) and the standards organizations identified as Issuing Agencies (e.g., GS1, HIBCC, ICCBBA). Some of the common features are below.
UDI is a unique numeric or alphanumeric code composed of the following:
Device Identifier (DI): mandatory, fixed portion of UDI that identifies the labeler as well as the specific model or version of the device.
Production Identifier(s) (PI): conditional, variable portion of a UDI that identifies one or more of the following production information:
Serial number
Lot or batch number
Expiration date
Manufacturing date
Distinct identification code for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
Example Linear UDI Barcode
Example 2D Data Matrix UDI Code
A medical device labeler is required to present the UDI on labels and packaging in two formats:
Human-readable plain-text
Machine-readable format using automatic identification and data capture (AIDC) technology (e.g., barcode or 2D DataMatrix code)

What Is the Status of Global UDI?
The data collected for UDI Systems are far more than just the Device Identifier and the Production Identifiers—it can include over 100 data elements, depending on the device type and regional requirements. Navigating these differences requires extensive and ongoing regulatory intelligence and awareness of compliance requirements across worldwide jurisdictions.
Many countries have established their own specific UDI regulations, including:
- United States
- European Union
- Australia
- China
- Brazil
- Taiwan
- South Korea
- Saudi Arabia
- Singapore
- Switzerland
And the list continues to grow as more countries adopt UDI frameworks to strengthen device identification and patient safety.
Learn more about UDI with Import IQ podcast and UDI Experts, hosted by Dawa Medical LLC’s Stephan Toupin.
Reference: FDA Unique Device Identification System (UDI System) https://www.fda.gov/UDI