EUDAMED UDI Compliance Deadline Delayed – Updated Timeline for 2025–2026
The European Commission has delayed the mandatory use of the EUDAMED UDI/Device Registration module. While it was expected to become required starting in January 2026, no publication confirming module functionality has yet appeared in the Official Journal of the EU (OJEU).
With the entry into force of Regulation (EU) 2024/1860 on July 9, 2024, a gradual rollout of individual EUDAMED modules is now legally enabled. Each module becomes mandatory 6 months after a Commission notice is published in the OJEU confirming its functionality. This regulatory change, paired with recent MDCG guidance, shifts the focus from full-database readiness to module-level compliance milestones.
EUDAMED Module Rollout Timeline
The European Commission’s latest update (July 2025) anticipated the OJEU publication of functional modules that month, which would have triggered mandatory use in January 2026. However, that publication has not occurred as of late July 2025.
A hotfix to the EUDAMED system (v2.15.1) was released on July 22, 2025, signaling continued updates and pre-launch stabilization, but not formal declaration of readiness.
Below is the latest official timeline published by the European Commission:
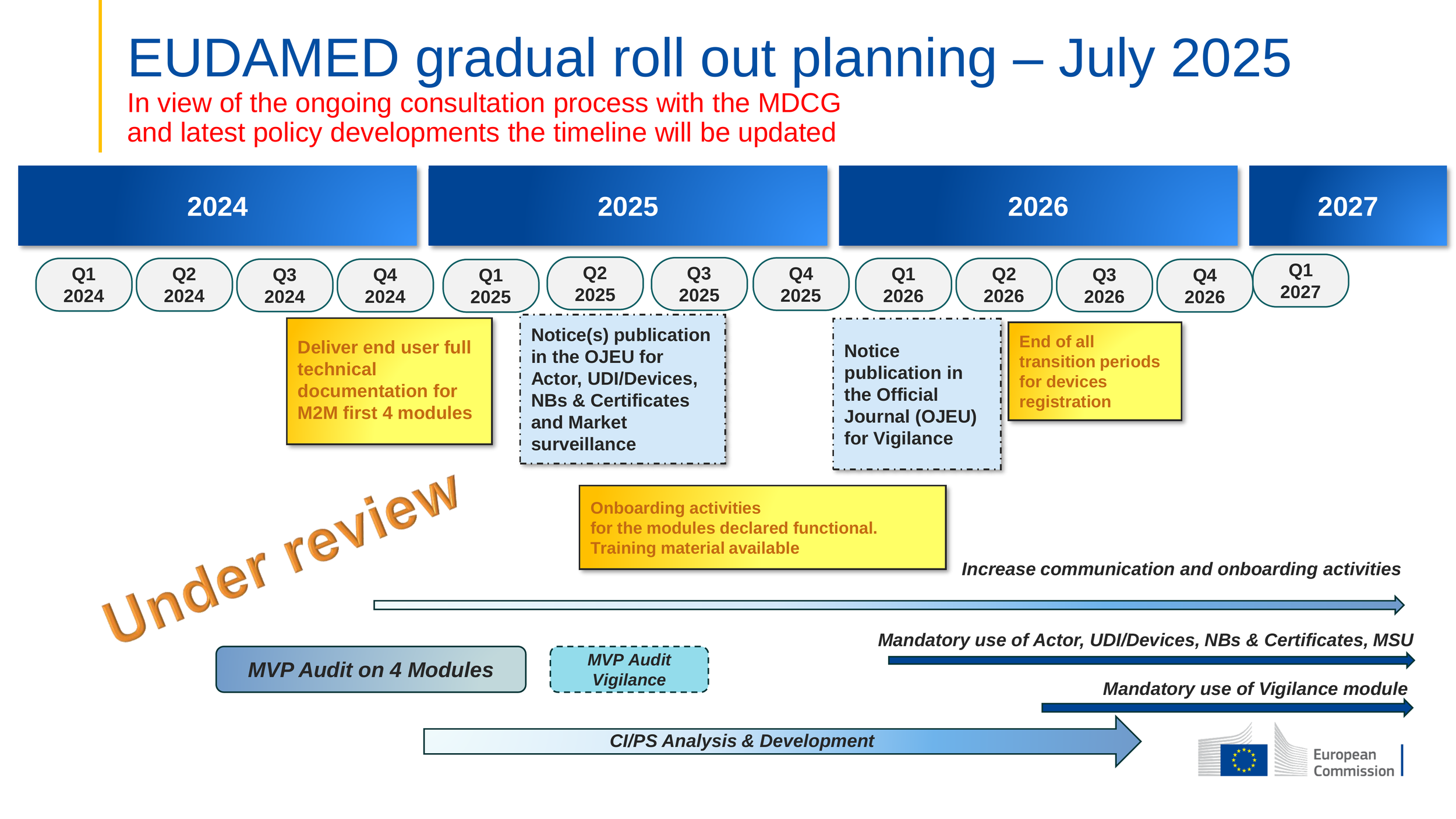
Source: European Commission – Updated Timeline for EUDAMED Roll-Out
Note: Once a module is declared functional in the OJEU, relevant obligations under MDR/IVDR become mandatory after a six-month transition period (per Articles 123(3)(d) and 113(3)(e)).
What This Means for You
- If you're not yet uploading UDI and device registration data to EUDAMED, now is the time to validate your data structure and prepare for submission.
- Don’t wait for the OJEU publication. Industry leaders are preparing now to avoid bottlenecks in early 2026.
- Legacy devices will likely need to be registered within 12 months of the applicable module becoming mandatory.
How UDI Experts Can Help
- Proactive Data Preparation: Structure and validate device, labeler, and economic operator data.
- Regulatory Readiness: Align internal systems with EUDAMED technical specs and validation rules.
- Timeline Strategy: Create an action plan to comply with each module as its use becomes mandatory.
Contact us today to assess your readiness and avoid last-minute compliance risks.
