Official EUDAMED Compliance Date — What You Need To Know
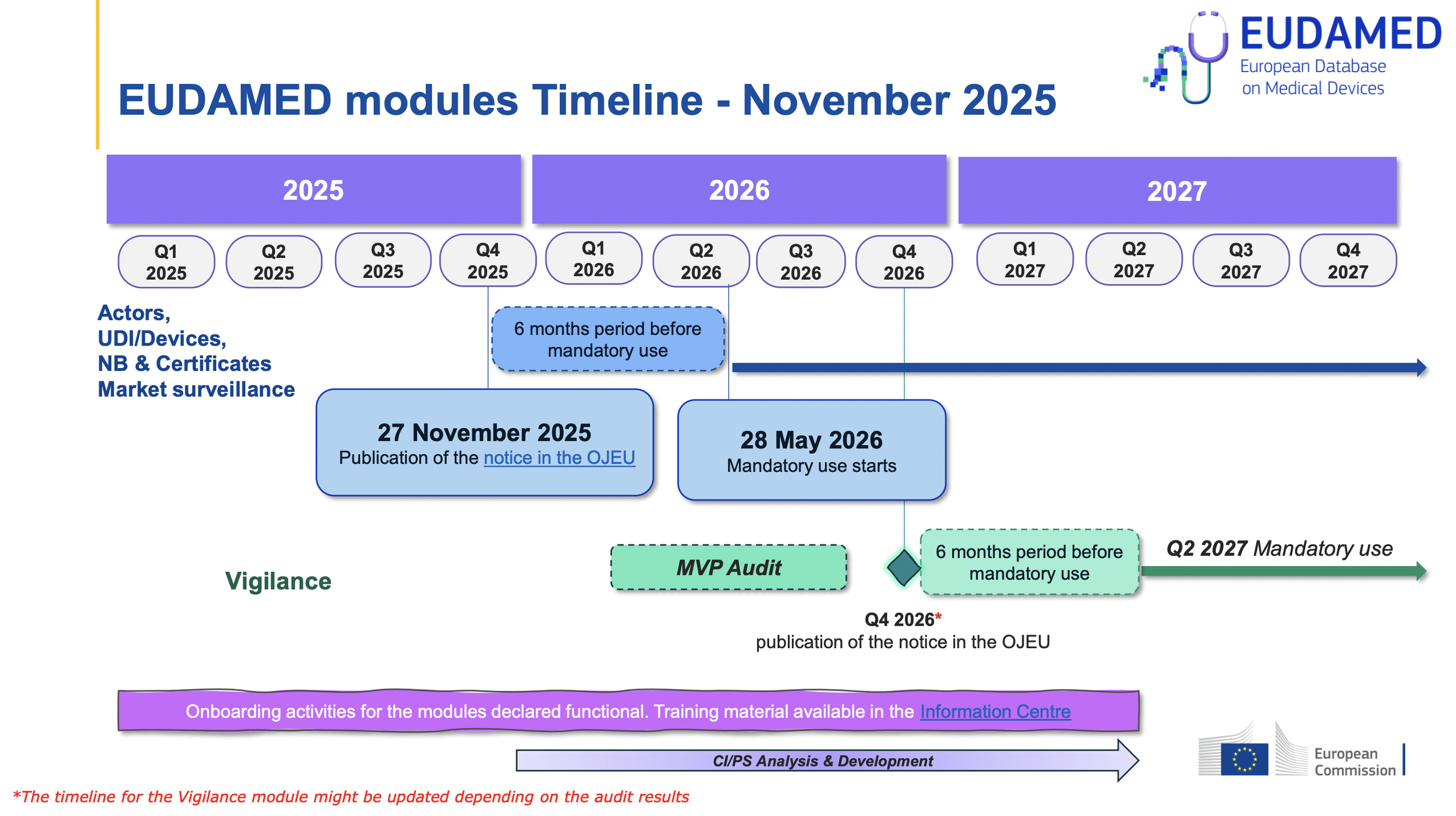
As of November 27th, 2025, The Official Journal of the EU has published a notice that 4 modules of EUDAMED have been audited with successful results.
These modules are:
Actor Registration
UDI/Device Registration
Notified Bodies and Certificates
Market Surveillance
What does this mean?
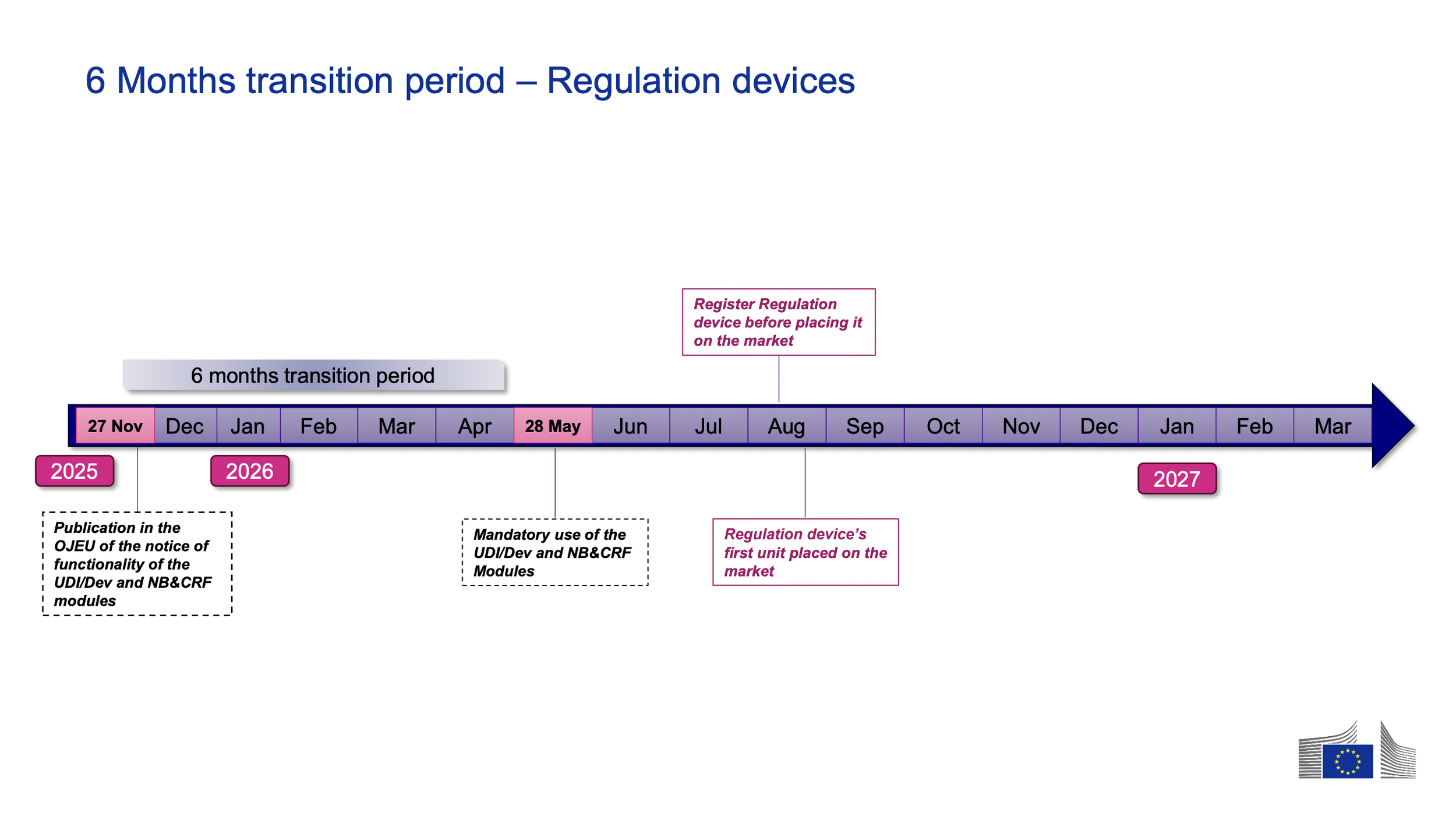
This means that starting May 28th, 2026, the first four EUDAMED modules listed above will be mandatory, following the 6 month transition period.
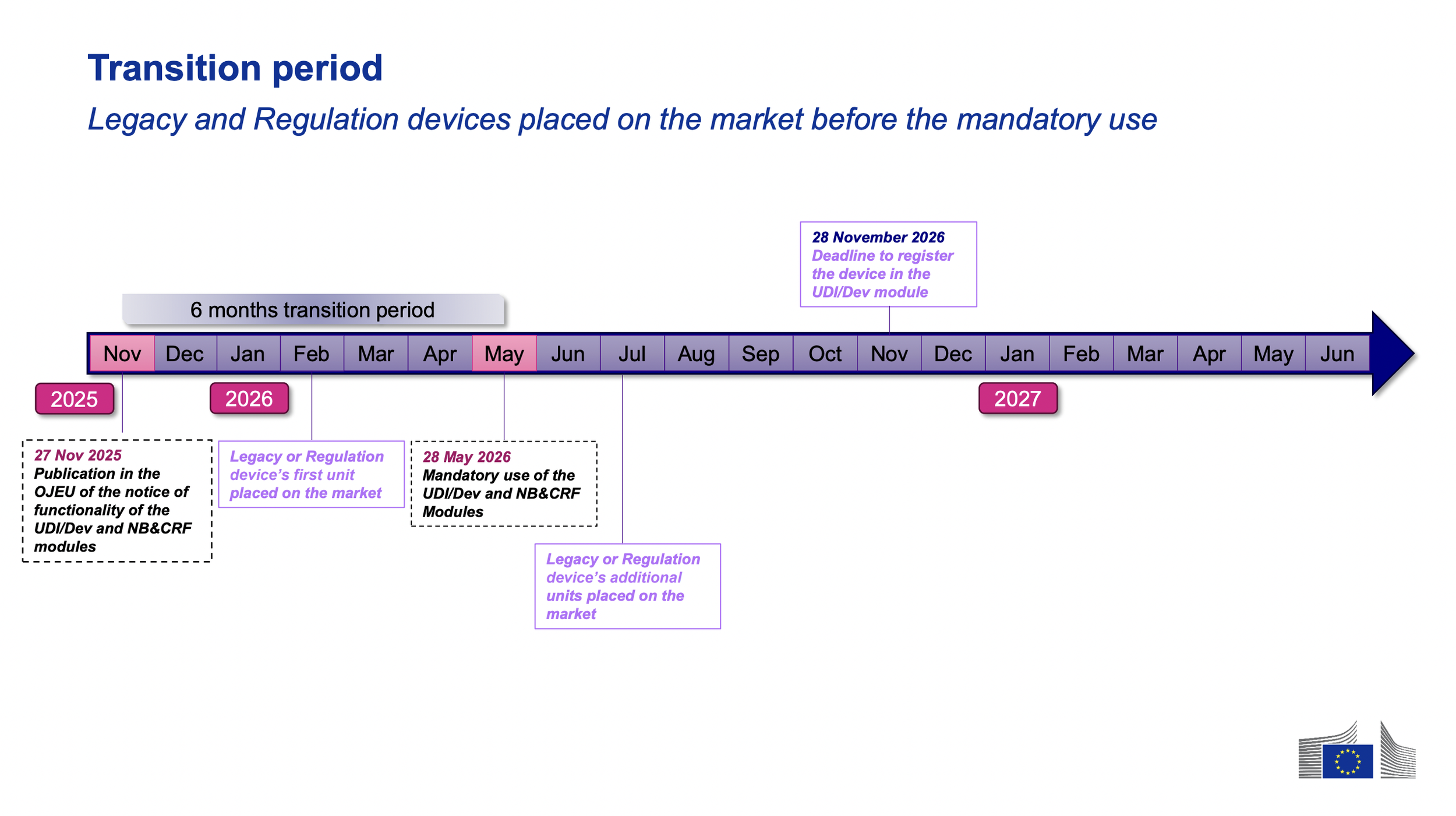
MDR devices placed on the market 6 months for the first time after the compliance date (May 28th, 2026 and beyond) must have the devices’ UDIs registered in EUDAED before placing the device on the market.
MDD and MDR devices placed on the market for the first time before the May 28th compliance start date, with additional units placed on the market after the compliance start date, must have the associated UDIs registered in EUDAMED as of November 28th, 2026.
Any MDD or MDR devices placed on market only before the May 28th, 2026 compliance date, in which these devices are not intended to be placed on the market as of May 2026 and beyond, are not required to be registered in EUDAMED, unless there is a vigilance activity that has occurred as of Q2 2027.
Have questions or need to get your devices registered in EUDAMED? Get your free consultation today!
Sources:
https://health.ec.europa.eu/medical-devices-eudamed/overview_en